MGH PDF Report
Challenge: A medical report needed a template update to show all critical information in a condensed and scannable format.
All content shown is test content. PHI has been removed.
Background
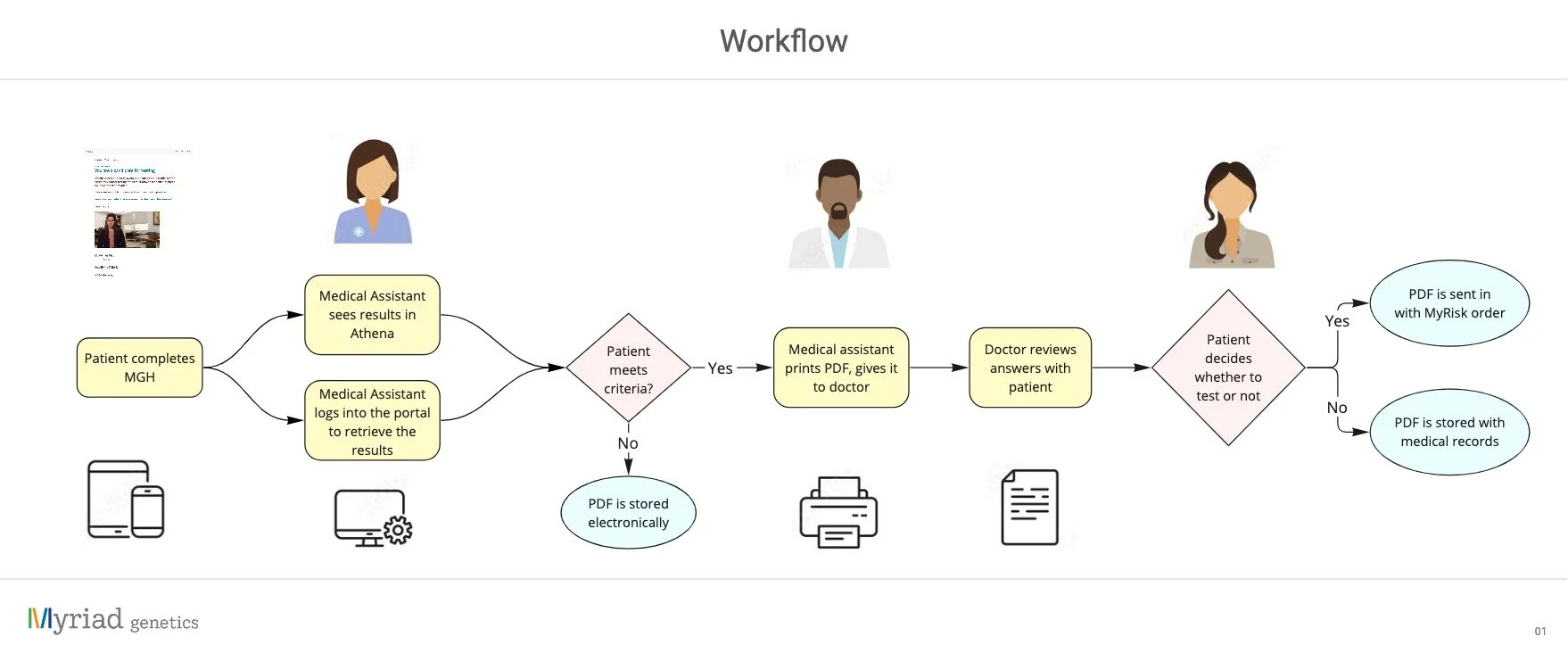
MyGeneHistory (MGH) collects medical history from patients and sends it to their medical provider. The provider’s staff view and print the report for the doctor, who uses it to determine the patient’s eligibility for genetic testing.
The development team was due to upgrade the system to a more dynamic setup, so I took the opportunity to update the design as well.
Process
The project started with a discovery phase, where I interviewed providers to chart their process and gather their feedback on the existing PDF. I also met with the development team to gather the technical requirements for the new design.
View discovery insights (PDF, 3.19MB)
During the design phase, I audited all the content across different versions of the PDF report, including additions that needed to be made. I outlined the content in a hierarchy based on provider needs, which I entered into Figma as a wireframe. The final design steps were to apply color and branding for a comp and to get internal stakeholder feedback.
Once the comp had been cleared internally, I showed the new design to providers to get their final thoughts. With a few tweaks, the new design was ready to pilot.
Team and Tools
I performed both UX research and design for this project. I partnered with a product manager and developers to make sure that the finished product met all necessary requirements.
Prototypes were constructed in Figma.
When installed, the finished template will be rendered as HTML and populated with patient data. It will then be converted to a PDF for consistency when viewed on the portal and printed.
Results
Usability studies showed a better response to the new design. Participants noted improvements to its clarity and visual hierarchy, clearly outlining all important information on the cover sheet. The addition of the “Guidelines” section, showing specific reasons why patients met testing criteria, was popular with participants.
The new design is due to move to pilot in Q3 2023.